what mass of chlorine gas mustbe reacted to form 4.5 g of phosgene
"COCl2" redirects here. For the compound CoCltwo, see Cobalt(II) chloride.
Non to be confused with phosphine, phosphene, oxalyl chloride, or phosgene oxime.
| align="center" style="background:lime;" | 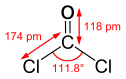 |  | |
| Names | ||
|---|---|---|
| IUPAC nameExpression error: Unexpected > operator. Carbonyl dichloride | ||
| Other names CG; carbon dichloride oxide; carbon oxychloride; Chloroformyl chloride; dichloroformaldehyde; dichloromethanone | ||
| Identifiers | ||
| CAS Number | 75-44-v | |
| ChEBI | CHEBI:29365 | |
| ChemSpider | 6131 | |
| EC Number | 200-870-3 | |
| Jmol 3D model | Interactive image | |
| PubChem | 6371 | |
| RTECS number | SY5600000 | |
| UNII | 117K140075 | |
| United nations number | 1076 | |
| InChI
| ||
| SMILES
| ||
| Backdrop | ||
| Tooth mass | 98.92 g mol−1 | |
| Appearance | colorless gas | |
| Density | four.248 grand/L (15 °C, gas) 1.432 k/cm3 (0 °C, liquid) | |
| Melting point | ||
| Boiling point | ||
| Solubility in h2o | decomposes in water[2] | |
| Solubility | soluble in benzene, toluene, acetic acid decomposes in booze and acid | |
| Structure | ||
| Molecular shape | Planar, trigonal | |
| Dipole moment | one.17 D | |
| Hazards | ||
| EU classification (DSD) | Very toxic (T+) | |
| R-phrases | R26 R34 | |
| S-phrases | (S1/2) S9 S26 S36/37/39 S45 | |
| NFPA 704 | 0 four 1 | |
| Flash point | ||
| Threshold Limit Value | 0.1 ppm | |
| Related compounds | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | ||
| | ||
| Infobox references | ||
Phosgene is the chemical compound with the formula COCl2. This colorless gas gained infamy every bit a chemical weapon during World War I. It is too a valued industrial reagent and building block in synthesis of pharmaceuticals and other organic compounds. In depression concentrations, its odour resembles freshly cut hay or grass.[iii] In addition to its industrial product, small amounts occur naturally from the breakup and the combustion of organochlorine compounds, such every bit those used in refrigeration systems.[four] The chemical was named by combining the Greek words 'phos' (meaning light) and genesis (birth); it does non hateful it contains any phosphorus (cf. phosphine).
Contents
- 1 Structure and basic properties
- 2 Production
- ii.1 Adventitious occurrence
- iii Uses
- 3.1 Synthesis of carbonates
- 3.2 Synthesis of isocyanates
- 3.three Laboratory uses
- 4 Other chemistry
- five History
- five.1 Chemical warfare
- 6 Safety
- 7 Encounter also
- eight References
- 9 External links
Structure and basic backdrop
Phosgene is a planar molecule as predicted by VSEPR theory. The C=O altitude is i.xviii Å, the C—Cl altitude is one.74 Å and the Cl—C—Cl bending is 111.viii°.[five] It is ane of the simplest acid chlorides, being formally derived from carbonic acid.
Production
Industrially, phosgene is produced past passing purified carbon monoxide and chlorine gas through a bed of porous activated carbon, which serves as a catalyst:[4]
- CO + Cl2 → COCl2 (ΔHrxn = −107.6kJ/mol)
The reaction is exothermic, therefore the reactor must be cooled. Typically, the reaction is conducted between 50 and 150 °C. Above 200 °C, phosgene reverts to carbon monoxide and chlorine, Keq (300K) = 0.05. World production of this compound was estimated to be 2.74 million tonnes in 1989.[4]
Because of safety issues, phosgene is nigh always produced and consumed within the same plant and extraordinary measures are fabricated to incorporate this toxic gas. Information technology is listed on schedule 3 of the Chemical Weapons Convention: All product sites manufacturing more than xxx tonnes per year must be declared to the OPCW.[6] Although less dangerous than many other chemic weapons, such equally sarin, phosgene is still regarded equally a viable chemical warfare agent considering information technology is and so like shooting fish in a barrel to manufacture when compared to the production requirements of more than technically advanced chemical weapons such as the showtime-generation nerve amanuensis tabun.[7]
Adventitious occurrence
Upon ultraviolet (UV) radiation in the presence of oxygen, chloroform slowly converts into phosgene by a radical reaction. To suppress this photodegradation, chloroform is oft stored in brown-tinted drinking glass containers. Chlorinated compounds used to remove oil from metals, such as automotive brake cleaners, are converted to phosgene by the UV rays of arc welding processes.[eight]
Phosgene may likewise be produced during testing for leaks of older-style refrigerant gases. Chloromethanes (R12, R22 and others) were formerly leak-tested in situ by employing a small gas torch (propane, butane or propylene gas) with a sniffer tube and a copper reaction plate in the flame nozzle of the torch. If any refrigerant gas was leaking from a piping or joint, the gas would be sucked into the flame via the sniffer tube and would cause a colour alter of the gas flame to a brilliant greenish blueish. In the process, phosgene gas would be created due to the thermal reaction. No valid statistics are available, but anecdotal reports suggest that numerous refrigeration technicians suffered the effects of phosgene poisoning due to their ignorance of the toxicity of phosgene, produced during such leak testing.[ citation needed ] Electronic sensing of refrigerant gases phased out the use of flame testing for leaks in the 1980s. Similarly, phosgene poisoning is a consideration for people fighting fires that are occurring in the vicinity of freon refrigeration equipment, smoking in the vicinity of a freon leak, or fighting fires using halon or halotron.
Uses
The smashing majority of phosgene is used in the production of isocyanates, the most important being toluene diisocyanate (TDI) and methylene diphenyl diisocyanate (MDI). These isocyanates are precursors to polyurethanes.
Synthesis of carbonates
Pregnant amounts are also used in the production of polycarbonates past its reaction with bisphenol A.[iv] Polycarbonates are an important class of applied science thermoplastic found, for example, in lenses in centre spectacles. Diols react with phosgene to give either linear or cyclic carbonates (R = H, alkyl, aryl):
- HOCRii-10-CR2OH + COClii → one/n [OCRtwo-X-CR2OC(O)-]n + 2 HCl
Synthesis of isocyanates
The synthesis of isocyanates from amines illustrates the electrophilic character of this reagent and its use in introducing the equivalent of "CO2+":[9]
- RNH2 + COCl2 → RN=C=O + 2 HCl (R = alkyl, aryl)
Such reactions are conducted in the presence of a base such as pyridine that absorbs the hydrogen chloride.
Laboratory uses
In the research laboratory phosgene still finds limited use in organic synthesis. A variety of substitutes take been developed, notably trichloromethyl chloroformate ("diphosgene"), a liquid at room temperature, and bis(trichloromethyl) carbonate ("triphosgene"), a crystalline substance.[ten] Aside from the higher up reactions that are widely skilful industrially, phosgene is also used to produce acrid chlorides and carbon dioxide from carboxylic acids:
- RCO2H + COClii → RC(O)Cl + HCl + CO2
Such acrid chlorides react with amines and alcohols to requite, respectively, amides and esters, which are commonly used intermediates. Thionyl chloride is more unremarkably and more safely employed for this application. A specific application for phosgene is the product of chloroformic esters:
- ROH + COCl2 → ROC(O)Cl + HCl
Other chemistry
Although information technology is somewhat hydrophobic, phosgene reacts with water to release hydrogen chloride and carbon dioxide:
- COCl2 + HtwoO → CO2 + ii HCl
Analogously, with ammonia, 1 obtains urea:
- COCl2 + 4 NH3 → CO(NH2)2 + 2 NHivCl
Halide exchange with nitrogen trifluoride and aluminium tribromide gives COF2 and COBr2 , respectively.[4]
History
Phosgene was synthesized by the British chemist John Davy (1790–1868) in 1812 by exposing a mixture of carbon monoxide and chlorine to sunlight. He named it "phosgene" in reference of the use of light to promote the reaction; from Greek, phos (low-cal) and factor (born).[11] It gradually became of import in the chemical industry as the 19th century progressed, particularly in dye manufacturing.
Chemic warfare
Following the all-encompassing use of phosgene gas in combat during World State of war I, information technology was stockpiled by diverse countries equally part of their secret chemical weapons programs.[12] [13] [14]
In May 1928, eleven tons of phosgene escaped from a state of war surplus store in central Hamburg.[15] 300 people were poisoned of whom x died.[15]

US Regular army phosgene identification poster from Earth War 2
Phosgene was so only frequently used by the Imperial Japanese Ground forces confronting the Chinese during the Second Sino-Japanese War.[16] Gas weapons, such as phosgene, were produced past Unit 731 and authorized past specific orders given past Hirohito (Emperor Showa) himself, transmitted by the primary of staff of the army. For example, the Emperor authorized the employ of toxic gas on 375 split occasions during the boxing of Wuhan from Baronial to Oct 1938.[17]
Safety
Phosgene is an insidious poisonous substance equally the smell may non exist noticed and symptoms may be tiresome to appear.[18] The odor detection threshold for phosgene is 0.iv ppm, iv times the threshold limit value. Its high toxicity arises from the action of the phosgene on the proteins in the pulmonary alveoli, the site of gas substitution: their damage disrupts the claret-air barrier, causing suffocation. It reacts with the amines of the proteins, causing crosslinking by germination of urea-like linkages, in accord with the reactions discussed in a higher place. Phosgene detection badges are worn by those at risk of exposure.[4]
Sodium bicarbonate may exist used to neutralise liquid spills of phosgene. Gaseous spills may exist mitigated with ammonia.[19]
Encounter also
- Diphosgene
- Triphosgene
- Bhopal disaster
- Carbonyl fluoride
- Carbonyl bromide
- Formaldehyde
References
- ↑ Merck Alphabetize , 11th Edition, 7310.
- ↑ http://www.inchem.org/documents/icsc/icsc/eics0007.htm
- ↑ CBRNE - Lung-Dissentious Agents, Phosgene May 27, 2009
- ↑ 4.0 4.one 4.two 4.3 4.4 4.5 "Ullmann'due south Encyclopedia of Industrial Chemical science". Phosgene (cylinder). Weinheim: Wiley-VCH. 2005. Digital object identifier:10.1002/14356007.a19_411. http://www.inchem.org/documents/icsc/icsc/eics0007.htm.
- ↑ Nakata, G.; Kohata, G.; Fukuyama, T.; Kuchitsu, K. (1980). "Molecular Structure of Phosgene equally Studied by Gas Electron Diffraction and Microwave Spectroscopy. The rz Structure and Isotope Effect". pp. 105–117. Digital object identifier:ten.1016/0022-2852(80)90314-8.
- ↑ Annex on Implementation and Verification ("Verification Addendum")
- ↑ https://itportal.decc.gov.united kingdom/cwc_files/S2AAD_guidance.pdf
- ↑ "Mutual Cleaners Can Turn Into Poison Gas". American Iron Magazine . TAM Communications. http://www.brewracingframes.com/id75.htm . Retrieved 14 October 2011.
- ↑ "p-Nitrophenyl Isocyanate". 1943. http://www.orgsyn.org/demo.aspx?prep=CV2P0453. ; "Coll. Vol.". pp. 453.
- ↑ Hamley, P. "Phosgene" Encyclopedia of Reagents for Organic Synthesis, 2001 John Wiley, New York. doi: 10.1002/047084289X.rp149
- ↑ John Davy (1812). "On a Gaseous Chemical compound of Carbonic Oxide and Chlorine". pp. 144–151. Digital object identifier:10.1098/rstl.1812.0008. JSTOR 107310.
- ↑ Base's phantom war reveals its secrets, Lithgow Mercury, 7/08/2008
- ↑ Chemical warfare left its legacy, Lithgow Mercury, 9/09/2008
- ↑ Chemical bombs sit down metres from Lithgow families for 60 years, The Daily Telegraph, September 22, 2008
- ↑ 15.0 xv.1 Ryan, T.Anthony (1996). Phosgene and Related Carbonyl Halides. Elsevier. pp. 154–155. ISBN 0444824456.
- ↑ Yuki Tanaka, "Poisonous substance Gas, the Story Japan Would Like to Forget", Bulletin of the Atomic Scientists, October 1988, p. sixteen–17
- ↑ Y. Yoshimi and S. Matsuno, Dokugasusen Kankei Shiryô II, Kaisetsu, Jugonen Sensô Gokuhi Shiryoshu, 1997, p. 27–29
- ↑ Borak J., Diller W. F. (2001). "Phosgene exposure: mechanisms of injury and treatment strategies". pp. 110–nine. Digital object identifier:10.1097/00043764-200102000-00008. PMID 11227628.
- ↑ "Phosgene: Health and Safety Guide". International Programme on Chemical Safe. 1998. http://www.inchem.org/documents/hsg/hsg/hsg106.htm.
External links
- Davy'due south account of his discovery of phosgene
- International Chemical Prophylactic Carte du jour 0007
- CDC - Phosgene - NIOSH Workplace Safety and Health Topic
- NIOSH Pocket Guide to Chemical Hazards
- U.S. CDC Emergency Preparedness & Response
- U.S. EPA Acute Exposure Guideline Levels
- Regime For Schedule 3 Chemicals And Facilities Related To Such Chemicals, OPCW website
- CBWInfo website
- Use of Phosgene in WWII and in modern-day warfare (Refer to Section 4.C of the article)
- An experience with accidental poisoning by heated tetrachlorethylene solvent
Source: https://military-history.fandom.com/wiki/Phosgene

0 Response to "what mass of chlorine gas mustbe reacted to form 4.5 g of phosgene"
Post a Comment